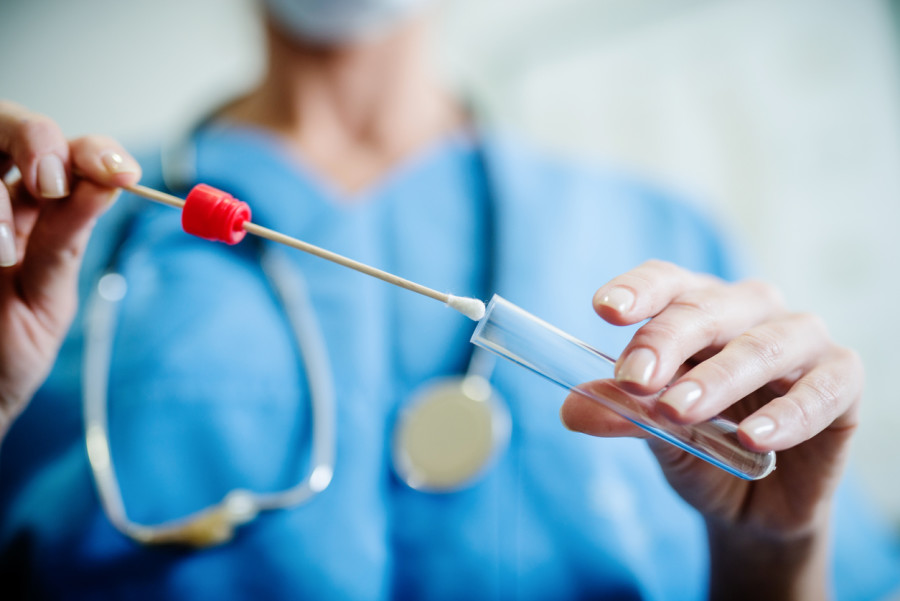
To combat the STD epidemic in the United States, the Food and Drug Administration has authorised the first-ever at-home diagnostic tests for chlamydia and gonorrhoea.
The recently authorised test instrument is administered in lieu of requiring individuals to travel; rather, it operates on the basis of a health questionnaire completed by the patient and reviewed by a physician.
Following this, the specimen is personally dispatched to the patient for personal use, and is subsequently returned to the laboratory via mail for analysis.
Introducing Simple 2 Test by LetsGetChecked
Marketing the Simple 2 Test will be LetsGetChecked, a New York-based company that specialises in laboratory testing. This service entails individuals submitting samples to a lab from the convenience of their residences. The lab then delivers the results in a secure online format.
Prescription-Free Convenience and Preventive Care
The new test will be available without a prescription. It spares individuals the inconvenience of invasive in-office examinations and facilitates treatment initiation prior to the exacerbation of infections.

Federal regulators hailed this development as a triumph for public health. It should help fight the rising frequency of STDs in the US. Access to routine preventive care was severely limited during the pandemic, which exacerbated this alarming trend.
FDA’s Milestone Approval and Public Health Impact
The director of the Centre for Devices and Radiological Health at the FDA, Dr. Jeff Shuren, stated on Thursday, “This authorization represents a significant milestone in public health, providing patients with more information about their health in the comfort of their own homes.”
“Our continued support for increased consumer access to diagnostic tests advances our objective of bringing more health care into the home.”
Discreet Testing and Results Delivery
Following the antibody test for HIV, which enables at-home screening, this is the initial self-test approved by the FDA.
The Simple 2 test will be discreetly delivered to an individual’s residence for a payment of $99 USD. Patients must complete a health questionnaire that a doctor will review after receiving the equipment. They will then submit urine or vaginal swabs to detect gonorrhoea and chlamydia bacteria.
Accurate Results and Virtual Consultations
The company asserts that accuracy is ensured when users mail their kits to the same accredited laboratories that are utilised by hospitals and physician’s offices for no additional cost.
In two to five days, individuals will be able to access their results through a secure online portal. They may consult with a healthcare provider to discuss treatment options if the test is positive.
Risks and Follow-Up Support
According to the FDA, the primary risk associated with using the at-home test is receiving a false negative result, which “could lead to delays in effective treatment, progression to disseminated disease, and the spread of infection to other individuals in your community.”
If a positive test is followed by a doctor’s visit, the group can help the patient take their prescription.
The company stated that its services will also benefit the partners of those who test positive: “We can offer treatment for your partner(s) at an additional cost if your results are positive and you are deemed suitable for treatment.” Our team will join them after they start a video consultation to provide them piece of mind.
Contributors to STD Surge: Pandemic and Contraception Trends
As well as casual attitudes towards contraception, inadequate access to preventative care and disease screening during the pandemic were significant contributors to the rise in the incidence of sexually transmitted diseases.
As reported by the Centres for Disease Control and Prevention (CDC), the incidence of chlamydia, gonorrhoea, and syphilis rose to 2.53 million in 2021, consequently representing an almost 6 percent increase from 2020 and a 7 percent rise from 2017.
Condom Usage Decline and Women’s Role in Contraception
Since 2011, male condom use has increased between 75% and 55%, but now it is below 50%. There is a growing dependency on women to utilise contraception as a means of averting pregnancy.
According to the Office of Population Affairs’ annual family planning report, contraception does not prevent STDs.
Specifics of Incidence Increase in Gonorrhoea and Chlamydia
Between 2020 and 2021, men’s gonorrhoea incidence rose from 234.8 to 249.7 per 100,000. While it increased by over two percent among women from 173.8 to 177.9 per 100,000 women.
The incidence of Chlamydia, which was 1.58 million cases more prevalent in 2020, increased to 1.64 million in 2021.